2 octobre 2025
- Développement de technologies et procédés innovants dans le secteur cosmétique : de la plante tinctoriale au produit fini / Soutenance de thèse de Laura Guillouzo
Doctorante : Laura GUILLOUZO
Date et lieu : jeudi 02 octobre à 9h30 dans l’Amphithéâtre du Cerege du Technopôle de l'Arbois-Méditerranée (Batiment Pasteur, 13545, Aix-en-Provence)
Résumé : Afin d’offrir une alternative à la coloration d’origine pétrochimique dans l’industrie cosmétique, l’entreprise Le Rouge Français s’est tournée vers la coloration végétale. Un des défis majeurs pour les pigments issus de plantes tinctoriales est leur stabilité aux facteurs extérieurs lors de la manipulation et le stockage. Pour rester dans une démarche écologique, l’extraction au CO2 supercritique a été choisie dans ces travaux pour extraire les molécules responsables de la couleur des racines de garance puis d’étudier la faisabilité du procédé de Dispersion Séquentielle de Solution Saturée en Gaz (Sequential Dispersion Particles from Gas Saturated Solution SD-PGSS) pour la stabilisation des extraits obtenus. Ces procédés permettent d’extraire et d’encapsuler des composés d’intérêt sans solvant organique. Les encapsulats obtenus ont ensuite été intégrés à une formule de maquillage afin d’en étudier la stabilité.
Les études expérimentales de l'extraction de CO2 supercritique à partir de racines de garance - Rubia tinctorum L. - ont été réalisées sur différents volumes d'autoclave, à des pressions comprises entre 200 bar et 400 bar, à des températures comprises entre 40 °C et 60°C et à un débit de CO2 continu (0,14 kg/h pour l'échelle de laboratoire et 1,37 kg/h pour l'échelle semi- pilote). Les cinétiques d’extraction ont été modélisées à aux échelleslaboratoire et semi-pilote. Le rendement en colorant le plus élevé a été obtenu à 6,5 g/kg, à 400 bar et 60 °C. En ce qui concerne les teneurs en anthraquinones, les conditions optimales pour extraire des quantités maximales d'alizarine et de purpurine et minimales de lucidine, une molécule mutagène, étaient 300 bar et 60 °C. Une mise à l'échelle pilote a été réalisée à 60 °C, 200 bar et 240 bar, et à un débit de CO2 de 32 kg/h, afin de disposer d’une masse d’extrait suffisante pour être utilisée dans la formulation de rouges à lèvres et en vue d’une industrialisation future du procédé. La plus grande quantité d'extrait solide a été obtenue à 200 bar et 60 °C. Les extraits obtenus à échelle pilote ont été également utilisés ensuite pour l’étude d’encapsulation.
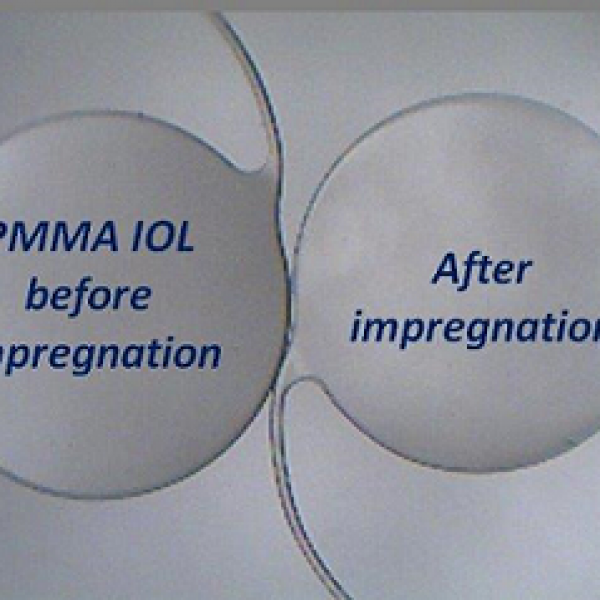
Pour l’encapsulation, le procédé SD-PGSS a été réalisé aux conditions opératoires de 150 bar et 65 °C avec l’acide arachidique comme excipient. La mise en oeuvre du procédé a conduit à l’obtention de rendements modérés (supérieurs à 60 %), avec des taux de chargement supérieurs à 90 % et des particules de diamètre moyen compris entre 0,7 μm et 11,4 μm. Les tests de stabilité réalisés en formulant des rouges à lèvres à partir d’encapsulats ont permis de montrer que l’encapsulation par SD-PGSS limite l’influence négatif du pH de la peau sur les pigments.
Mot clés : CO2 supercritique, extraction végétale, SD-PGSS, cosmétiques, plantes tinctoriales
Jury :
Mme Raphaëlle SAVOIRE, Rapporteure, Professeure, Bordeaux INP
M. Nabil GRIMI, Rapporteur, Professeur, Université de Technologie de Compiègne
M. Grégory CHATEL, Examinateur, Maître de conférence, Université de Savoie Mont-Blanc
M. Antoine LEYBROS, Examinateur, Cadre scientifique, CEA Marcoule
Mme Yasmine MASMOUDI, Examinatrice, Maîtresse de conférence, Aix-Marseille Université
M. Jérôme LABILLE, Président du jury, Directeur de recherche, CNRS CEREGE
Mme Elisabeth BADENS, Directrice de thèse, Professeure, Aix-Marseille Université
M. Adil MOUAHID, Co-encadrant de thèse, Maître de conférence, Aix-Marseille Université
Mme Elodie CARPENTIER, Membre invitée, Ingénieure, Société Le Rouge Français